Newsroom
Boston Scientific Receives FDA Approval for Next-Generation Image Guided Programming Software for Deep Brain Stimulation Therapy
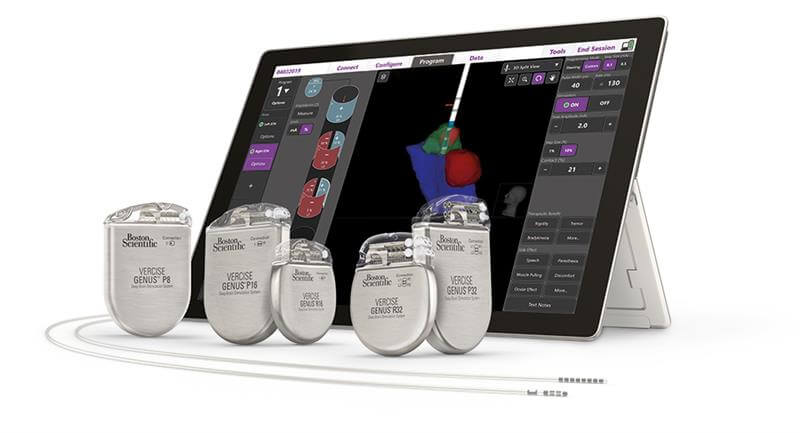
Boston Scientific, a global leader in medical technology, received U.S. Food and Drug Administration (FDA) approval for its latest image guided programming software, Vercise™ Neural Navigator with STIMVIEW™ XT. Developed in collaboration with Brainlab AG, STIMVIEW XT enables clinicians in real-time, the ability to visualize both lead placement and stimulation modeling of the brain anatomy of their patients living with Parkinson’s disease or essential tremor.
“Every person’s experience living with Parkinson’s disease or essential tremor is unique, and their treatment should be as unique,” said Maulik Nanavaty, Senior Vice President of Neuromodulation at Boston Scientific. “Our technologies enable clinicians to precisely see, shape and steer Deep Brain Stimulation therapy to meet their patients’ individual needs.”
For more information (https://news.bostonscientific.com/2022-04-12-Boston-Scientific-Receives-FDA-Approval)