Press Releases
Brainlab Announces CE Mark for ExacTrac Dynamic* Patient Positioning and Monitoring
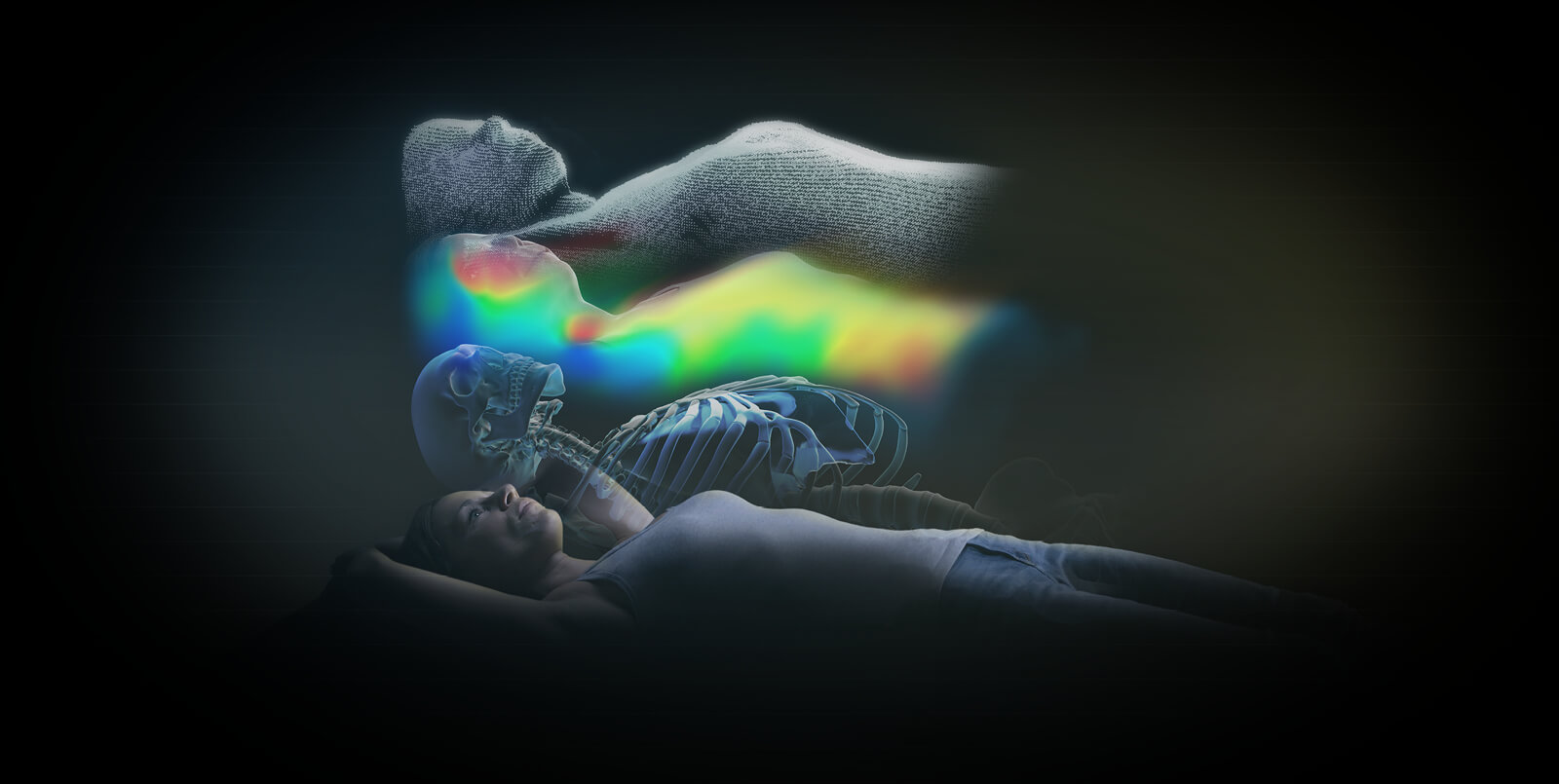
New 4D thermal surface tracking combined with flagship X-ray technology increases versatility**
MUNICH, April 21, 2020 – Brainlab, the digital medical technology company, today announced CE Mark (Conformité Européenne) approval for ExacTrac® Dynamic, the company’s next generation patient positioning and monitoring system. The new system offers never-before-seen, high-speed thermal surface tracking technology combined with an update of ExacTrac X-ray monitoring, providing advanced capabilities. New clinical workflows allow for treatment of a wide array of indications and together these advancements make ExacTrac Dynamic the all-in-one radiotherapy tracking solution.
ExacTrac Dynamic delivers several new value drivers while still delivering key benefits like high performance, non-coplanar X-ray monitoring and submillimetric precision***. Combined, the new system expands utility beyond legacy stereotactic radiosurgery (SRS) capabilities into surface-guided radiation therapy opportunities.
Advanced technology development and integration: ExacTrac Dynamic addresses a full range of patient positioning and monitoring requirements through consolidation of internal tracking and surface guidance technologies, including a novel thermal surface camera.
New 4D thermal camera: Submillimetric accuracy*** is delivered with a single high-speed stereoscopic video camera system which incorporates 300,000 3-D surface points to externally monitor patient position and detect movement during treatment. Each of the surface points acquired by the ExacTrac Dynamic structured light system are matched to a corresponding heat signal generated by this new thermal camera, creating another dimension in which to track the patient’s position consistently, with great accuracy and very low latency.
New 4Pi frameless immobilization*: ExacTrac Dynamic simplifies frameless fixation with three moldable thermoplastic mask options designed with patient comfort in mind. A user-centric, clipless design provides accessory-free immobilization options for most linac couch tops.
ExacTrac Dynamic X-Ray monitoring: Deep integration with linear accelerators from both Elekta and Varian Medical Systems allow positioning and monitoring workflows to function seamlessly, including automatic patient loading, gantry or MU triggered imaging, and automated beam-hold with repositioning from outside the treatment room. These high-precision tracking and verification capabilities are critical requirements in the delivery of extremely effective and high doses in precision radiotherapy.
“The CE Mark of ExacTrac Dynamic is a critical milestone toward future extra-cranial applications, including deep inspiration breath-hold and markerless tracking in the lung” said Stefan Vilsmeier, President and CEO, Brainlab. “These trying and uncertain times re-emphasize the importance of preserving as much lung function as possible.”
To learn more about, visit brainlab.com/radiosurgery-products/exactrac/.
About Brainlab
Brainlab, headquartered in Munich, develops, manufactures and markets software-driven medical technology, enabling access to advanced, less invasive patient treatments.
Core products center on information-guided surgery, radiosurgery, precision radiation therapy, digital operating room integration, and information and knowledge exchange. Brainlab technology powers treatments in radiosurgery and radiotherapy as well as numerous surgical fields including neurosurgery, ENT, CMF, spine and trauma.
Privately held since its formation in Munich, Germany in 1989, Brainlab has approximately 13,300 systems installed in 116 countries. Brainlab employs about 1,400 people in 19 offices worldwide, including around 430 Research & Development engineers, who form a crucial part of the product development team. www.brainlab.com
*FDA approval pending
**Compared to ExacTrac 6.5
***data on file