Webinar
Navigated Ultrasound in Neurosurgery - Ask the Experts
Sep 17, 2020

Description
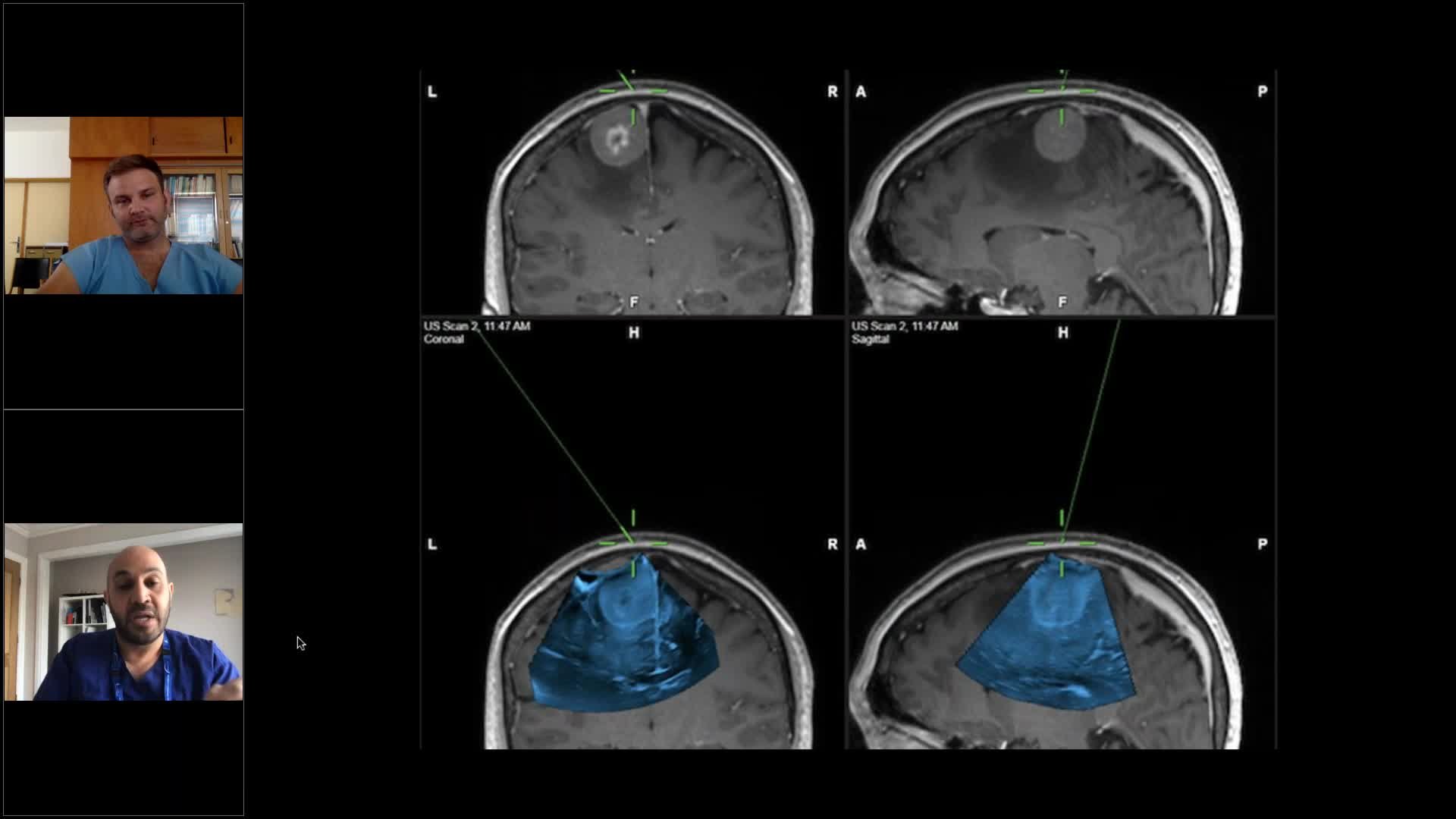
Brainlab invites you to a live webinar: “Navigated Ultrasound in Neurosurgery” on 17th September at 5:00 PM (AEST, GMT+10) hosted by Dr. Awad and Dr. Bartos, both experienced ultrasound users. Join their discussion around the applications of navigated ultrasound in neurosurgery, where they sharing their learning curve to fast track yours and answer your clinical application questions.
This webinar will cover topics, including:
– How to best start using ultrasound in neurosurgery?
– How to interpret intraoperative ultrasound images during the course of tumor resection?
– What to consider before and during surgery to optimize ultrasound usage?
– Q&A session with the audience.
We look forward to meeting you online!
Language | English
In case you can not join the webinar, it will be recorded and shared afterwards.
Speaker:

Mohammed Awad, MD, Neurosurgeon
The Royal Melbourne Hospital, Australia

Michael Bartos, MD
University Hospital Hradec Kralove, Czech Republic
See more upcoming webinars
Register now