Webinar
Augmented Reality-Assisted Surgery – The Benefits of Microscope Navigation in Neurosurgery
Feb 10, 2021
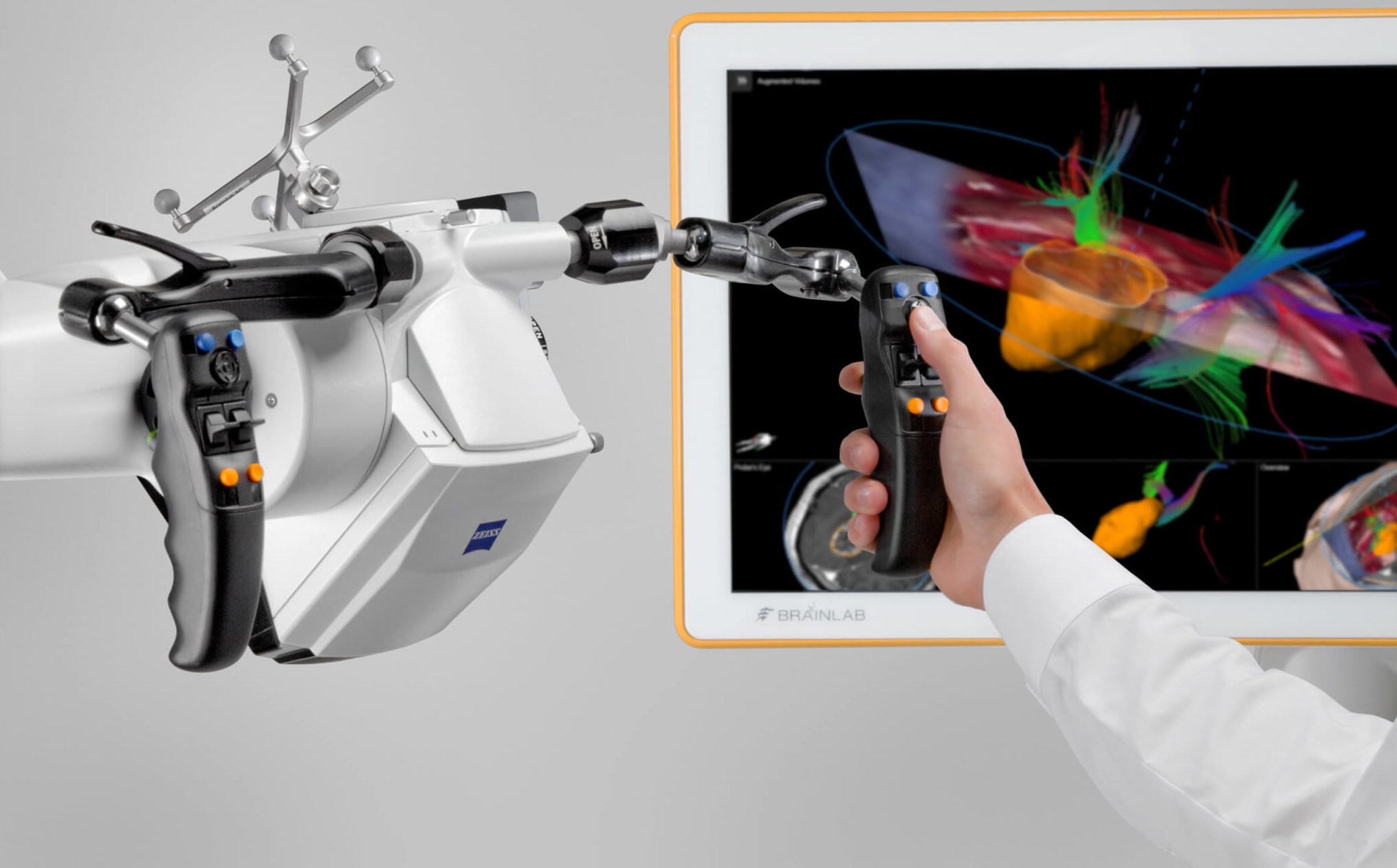
Descrição
Brainlab invites you to join our live webinar, “Augmented Reality-Assisted Surgery – The Benefits of Microscope Navigation in Neurosurgery” on February 10, 2021 at 4:00 PM CET. Presented by Margrét Jensdóttir, MD (Karolinska University Hospital, Stockholm, Sweden), Sabino Luzzi, MD, PhD (University of Pavia and Foundation IRCCS Policlinico San Matteo, Pavia, Italy) and Christian Raftopoulos, MD, PhD (Cliniques universitaires Saint-Luc, Brussels, Belgium), this webinar will also include a discussion and Q&A session.
The webinar will cover:
· Why and how to use augmented reality-assisted techniques in neurosurgery?
· The benefits of microscope navigation in tumor surgery
· The advantages of microscope navigation in vascular surgery
We look forward to meeting you online!
Participation is free of charge.
The views, information and opinions expressed during this webinar are the speakers’ own and do not necessarily represent those of Brainlab.
Palestrante:
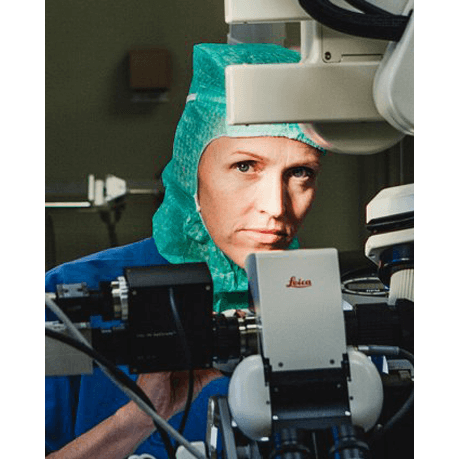
Margrét Jensdóttir, MD
Karolinska University Hospital,, Stockholm, Sweden

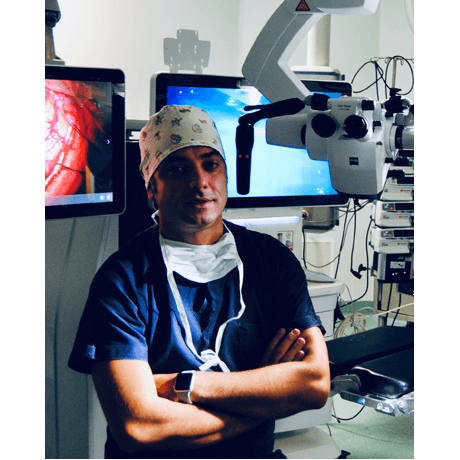
Sabino Luzzi, MD, PhD
University of Pavia and Foundation IRCCS Policlinico San Matteo, Pavia, Italy
Consultar mais webinars programados
Inscreva-se agora