Webinar
Computer-assisted Surgery for Reconstruction of Post-Traumatic Cranio-Orbital Deformity
Dec 15, 2020
Description
Brainlab invites you to join our live webinar, “Computer-assisted surgery for reconstruction of post-traumatic cranio-orbital deformity”, on December 15, 2020 at 4:00 PM CET presented by Michael P. Grant, MD, PhD, FACS is Chief of Plastic, Reconstructive and Maxillofacial Surgery at the R Adams Cowley Shock Trauma Center, University of Maryland Medical Center, and Professor of Surgery and Ophthalmology, University of Maryland School of Medicine in Baltimore Maryland.
This webinar will cover topics including:
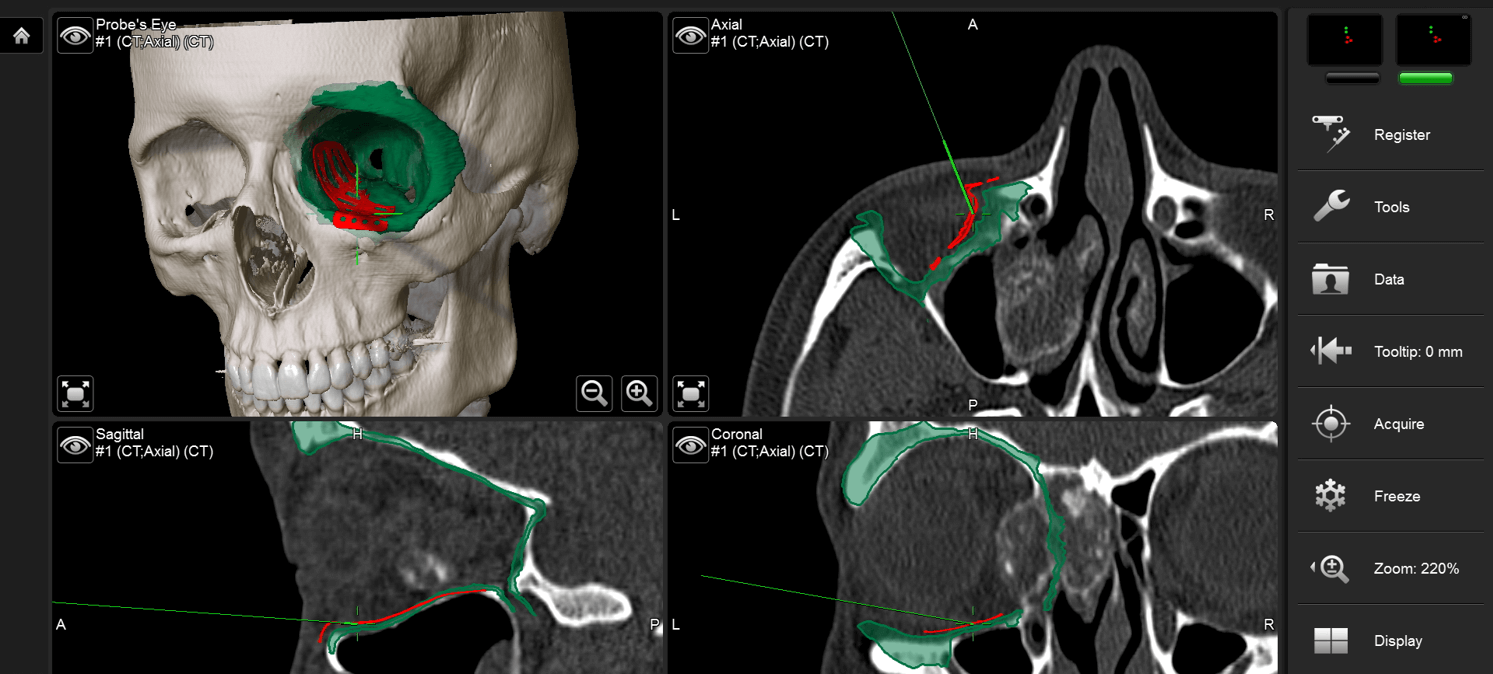
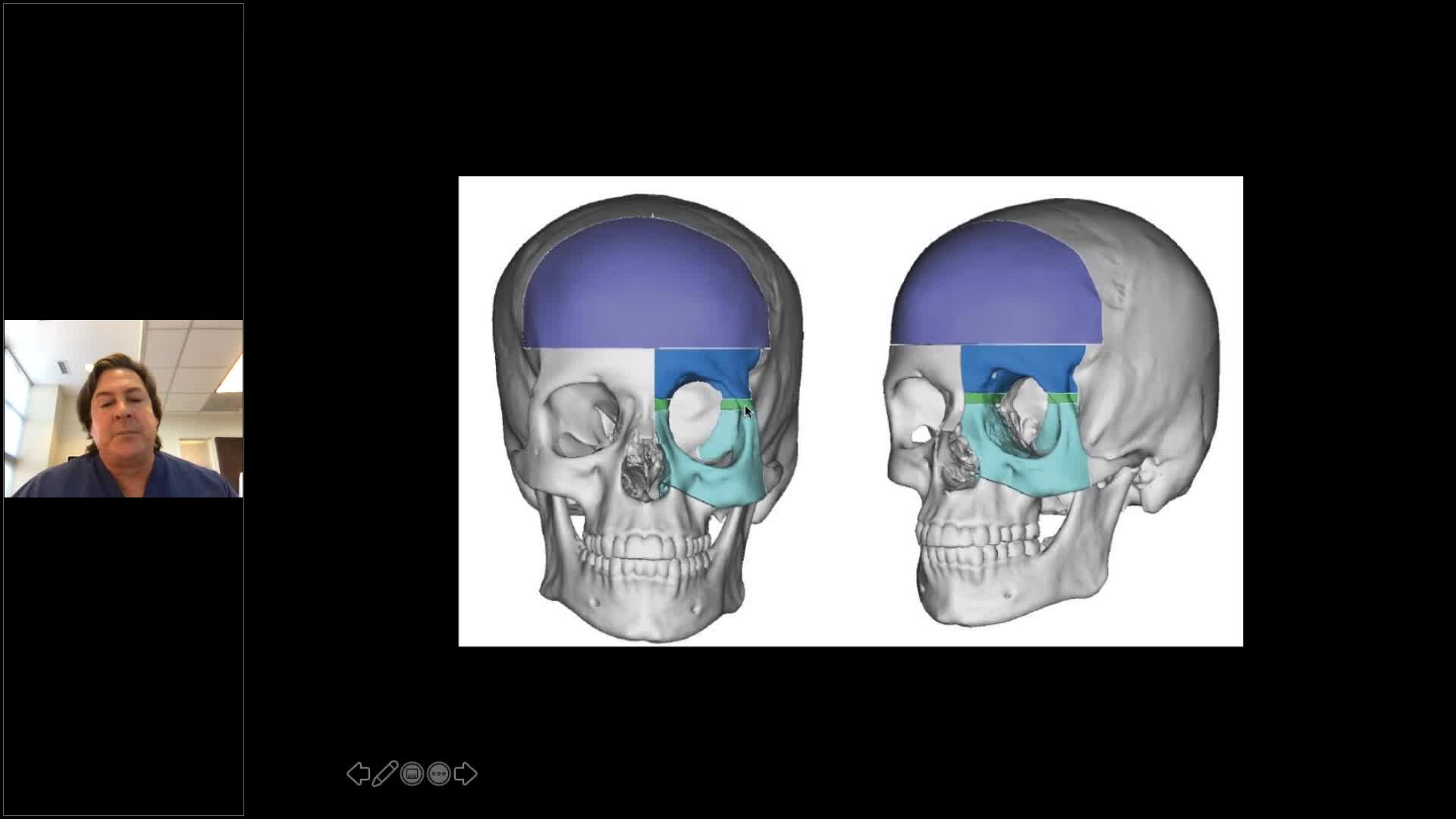
Learn to critically assess and diagnose post-traumatic defects of the frontal-orbital region
Understand the role of computer assisted surgical techniques in achieving optimal results
Learn to best evaluate the outcomes in these procedures, and avoid common complications
We look forward to meeting you online!
Language | English
In case you can not join the webinar, it will be recorded and shared afterward.
Participation is free of charge.
The views, information and opinions expressed within this presentation are from the speakers and do not necessarily represent those of Brainlab.
Speaker:

Michael P. Grant MD, PhD, FACS, Craniofacial Surgeon
Baltimore, USA
Moderator:

Jana Guggenberger, Director Product Management & Marketing Spinal/CMF Planning/Navigation
See more upcoming webinars
Register now